 |
Applying Analytical Chemistry to developing new methods and solving Technical Problems for the Lubricant Industry with superior Understanding and Technical Support. |
|
 |
|||
Root Cause AnalysisUsing Oil Analysis as a Tool in Fluid Degradation RCA Primary Tests for Fluid Degradation Fourier Transform Infrared Analysis (FTIR): ASTM E2412 - Infrared Spectroscopy is a method for measuring the chemistry of organic molecular components. FTIR is a tool that can be applied to monitor additive depletion, organic degradation by-products and the presence of various contaminants. It is a good primary test to measure chemistry changes of the fluid basestock, in addition to identifying what degradation mechanism may be responsible for the fluid degradation. Ultracentrifuge (UC), Sedimentation Rating – A method to isolate all of the insolubles in a sample. It is accomplished by spinning the sample at 20,000 RPM in a centrifuge for 30 minutes. The insolubles are separated from the fluid centrifugally allowing a visual sediment rating scale to be used. The minimum value of 1 represents no to low total insoluble levels. The maximum value of 8 represents a critical level of insolubles. Limitations include the inability of the test to differentiate between oil degradation by-products and other insoluble contaminants (dirt). The centrifugation process can also remove additives (VI improvers, Dispersants and Sulfonates) and can be laborious to run. Secondary Tests for Fluid Degradation Linear Sweep Voltammetry (LSV): ASTM D6971 – This test is designed to detect the oxidative health of a lubricant by measuring the primary antioxidants in the fluid. The level of remaining additive, and thus remaining useful life of the lubricant, are determined by comparison to original levels. It is possible to correlate the results of LSV to fluid degradation provided there is a significant amount of data from that particular fluid type. Rotating Pressure Vessel Oxidation Test (RPVOT): ASTM D2272 - (Previously RBOT). An important property of lubricating oil is its oxidation stability or resistance. The RPVOT test is a controlled, accelerated oxidation test of a lubricant used to measure the performance of remaining antioxidant additives. Results are evaluated and compared to new oil levels. This test has limited value as a primary test because fluid degradation can take place on isolated segments of the lubricant causing insolubles to be created without meaningful drops in RPVOT values. Therefore, it is not uncommon to see sludge and varnish problems occur in oils even with high RPVOT values. Acid Number: ASTM D974, D644 – This tool is a measure of the acidic constituents present within the lubricant. Most rust inhibitors used in oils are acidic and contribute to the acid number of the new oil. Increases from the new oil level are monitored, and for the most part, increases reflect the presence of acidic oxidation products. Though less likely, increases in acid number could be attributed to contaminants, mixtures of products, and/or chemical transformations. Although this is a valuable tool, it is looking at the production of chemistry that forms after a problem is already present. The test is inherently insensitive of weak organic acids, many of which are produced during lubricant degradation. Other Useful Analytical Tools in the RCA Process High Pressure Differential Scanning Calorimetry (HPDSC): ASTM D6186 - is a tool that is used to measure the oxidation induction time of lubricating oils under oxygen at 500 psig and elevated temperature (130 and 210°C). Many researchers feel that this tool can relate to RPVOT or RULER in its ability to test for the oxidation life expectancy of the lubricant. Because the sample size is very small, bulk solution effects are minimized and one is capable of observing good interchange of the sample with its atmospheric oxygen. This allows good repeatability of the test procedure with a reasonable reaction time. Gas Chromatography (GC/MS) - is a separation technique that is applied to the light-ends of a lubricant. It can be used to separate portions of the lubricant’s basestock, as well as many of the smaller additives. The refinery controls the production process of the basestock itself using this technique. When linked to a Mass Spectrometer detector, this tool can also identify the components that are being separated. The mass spectrometer detector can also increase the delectability of the compounds being separated. When using the GC/MS, one is capably of measuring the concentrations of additives, such as antioxidants, to as low as 10 ppm. Thermogravimetric Analysis TGA: D6370, D5967 - is a tool that measures the weight loss of a sample as it is heated in a controlled environment. The tool allows one to study the different components that are present by observing their vaporization temperature or decomposition points. Determination of the amounts of organics (oil, polymer), carbon black and ash in the sample can be identified. Flash Point: ASTM D92 – is a method to measure the minimum temperature at which an oil vapor will support combustion for a minimum of 5 seconds. Some forms of thermal degradation will produce light-ends that have the ability to lower the lubricant’s flash point. Flash point is a common test used to determine the presence of fuel dilution in a used lubricant. Dissolved Gas Analysis (DGA): ASTM D3612 – is an analytical test that measures the dissolved gasses in oils by gas chromography. Certain degradation mechanisms cause a lubricant’s hydrocarbon molecule to crack producing light end gasses that are entrained in the oil. The type and distribution of these gasses can give an important clue as to the degradation mechanism responsible. Particular gasses that are useful to examine when applying this test to fluid degradation are hydrocarbons (methane, ethane, ethylene, acetylene) carbon oxides (carbon monoxide, carbon dioxide) and hydrogen. Much research has been done to identify the specific gasses which are produced once the hydrocarbon molecule is cracked (temperatures in excess of 300oC). Acetylene for example occurs at temperatures above 1,000oC. Color: ASTM D1500 – Rapid color change of the fluid may indicate accelerated oil degradation, a mixture of oils in service, or contamination with another product and is an important characteristic to monitor over time. Oil darkening is often due to chromophores created in the degradation process. In addition, certain additives produce distinct color bodies after depletion. It is important to note that some fluid’s may have not undergone a color change and could still be degraded to dangerous levels, while the reverse is also true. Scanning Electron Microscope/Energy Dispersive X-Ray Spectrometer (SEM/EDS) – A high energy electron beam reflects off the surface of an object providing detailed three-dimensional visual observations, while also identifying the elemental composition of the object. This is a powerful tool in examining deposit formation, metallurgical analysis and failure analysis. Nuclear magnetic resonance spectroscopy (NMR) - is a tool similar to FTIR in that it allows one to study the molecular properties of the lubricant. By applying a magnetic field to the molecule, certain atoms yield a nuclear spectrum that is affected by its chemical environment. For oil analysis, the most commonly used atoms for which NMR spectra can be recorded are hydrogen (1H), carbon-13 (13C) and phosphorus (31P). NMR is closely related to MRI (Magnetic Resonance Imaging) most commonly used to capture images of soft tissue damage in human medicine. This tool will allow the research very detailed structural information about the sample’s chemistry. Gel permeation chromatography (GPC) - is a technique that physically separates the sample by the molecular size of its components. It is valuable for the study and separation of high molecular weight materials from the base oil, additives and formulated lubricants. These high molecular components include many of the oxidation condensation products. Spectrochemical Analysis: ASTM D6595, D5185 – is a tool to measure and monitor specific trace metals for wear and corrosion levels, airborne or internally generated contaminants, and certain additives. Particles detected are typically 8-mm and less and the results are reported in parts per million (ppm) by weight. Water Content: ASTM D6304, D1744 - Water contamination within oil systems adversely affects the lubricant by acting as a catalyst for oxidation and rapidly depleting water sensitive additives (also called “additive washout”). Water also promotes rusting, corrosion, micro-dieseling and filter plugging. Results are reported in parts per million (ppm) by weight. Particle Count: ISO 11171, NAS 1638 – is a test designed to count the number of particles present greater at a given micron sizes per unit volume of fluid. The results reflect the insoluble contaminants present within that size range and are applied to assess fluid cleanliness and filtration efficiency. Cleanliness levels are also represented by the ISO 4406 classification system to classify the particles larger than 4-mm, 6-mm, and 14-mm per milliliter of fluid. Degradation Mechanisms Oxidation – Oxidation is the reaction of materials with oxygen and the successive electron transfer processes that result. It can be responsible for viscosity increase, varnish formation, sludge and sediment formation, additive depletion, base oil breakdown, filter plugging, loss in foam properties, acid number increase, rust and corrosion. Controlling oxidation is a significant challenge in trying to extend the lubricant’s life. Many of the additives are formulated specifically to try to control the effects of oxidation. Some are specifically formulated to neutralize the reactive by-products produced from oxidation. There are no additives that can stop the oxidation process from occurring; they are just designed to oxidize first, in an attempt to protect the lubricant. Thermal Breakdown – In a mechanical working environment, the temperature of the lubricant is always a concern. In addition to separating the moving parts of the machinery, the lubricant’s job is also to dissipate generated heat. This means the lubricant will be heated – sometimes above its recommended stable temperature. The effects of this heating can cause the light ends of the lubricant to vaporize or the lubricant itself to decompose. The light end vaporization process will cause some additives to be removed from the system without doing their job, or the viscosity of the lubricant to increase. As the temperature exceeds the thermal stability point of the lubricant, molecules will actually crack apart, making smaller molecules. This thermal cracking (or thermal breakdown) can loose components, initiate further breakdown reactions, initiate polymerization, generate gaseous products, destroy additives and generate insoluble by-products. In some cases, thermal degradation will cause a decrease in viscosity. Micro-dieseling - Also known as pressure-induced thermal degradation, is a process in which an air bubble transitions from a low pressure zone to a high pressure zone resulting in adiabatic compression. This may produce localized temperatures in excess of 1,000oC resulting in the formation of carbonaceous by-products and accelerated oil degradation. Additive Depletion – Most additive systems are designed to be sacrificial in nature. They often transition into several intermediary stages during depletion. Monitoring additive levels is an important part of any condition monitoring program, not only to assess the health of the lubricant, but also to provide insights to lubricant degradation. Monitoring additive depletion can be quite complex depending upon the chemistry of the additive component. Electrostatic Spark Discharge – When clean, dry oil rapidly flows through tight clearances, internal friction within the oil can generate static electricity. The static electricity can accumulate and release a spark typically at sharp surfaces. The spark is estimated to be between 10,000 and 20,000oC. The most common place for this to occur is in mechanical filters. Contamination – Foreign substances in lubricants can greatly expedite lubricant degradation. Metals such as copper and iron are copper catalysts to the degradation process. Water and air can provide a large source of oxidation to react with the oil. It goes without saying that a contaminant-free lubricant is ideal and monitoring a fluid’s contamination levels provide significant insight to the machine health. 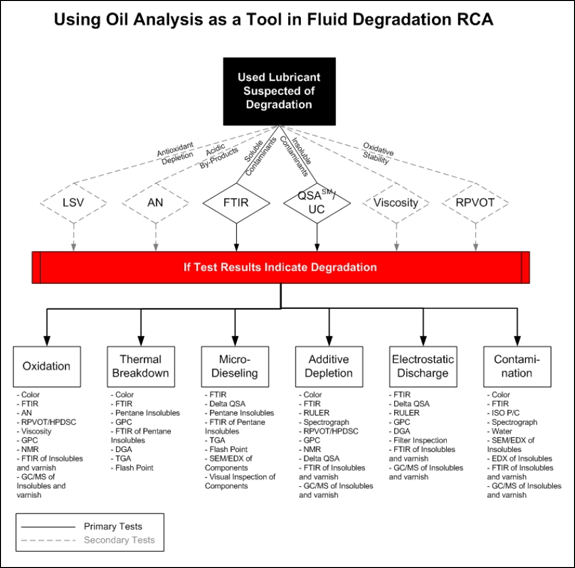
Figure 1. Analytical tools to assess lubricant degradation. |
|
|||||||||
| Copyright 2009 - Red Wheel Web Creations | Webmaster |